Mon, Nov 24, 2025
[Archive]
Volume 1, Issue 2 (10-2024)
IJHMD 2024, 1(2): 4-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yazdani S, Foroughi Z, Karimian Rad E, Haghdoost A, Jabali H, Hajikhani A et al . A Bayesian approach model to COVID-19 case definitions. IJHMD 2024; 1 (2) :4-10
URL: http://jhd.goums.ac.ir/article-1-44-en.html
URL: http://jhd.goums.ac.ir/article-1-44-en.html
Shahram Yazdani1 

 , Zeynab Foroughi2
, Zeynab Foroughi2 

 , Emad Karimian Rad *3
, Emad Karimian Rad *3 

 , Ali-Akbar Haghdoost4
, Ali-Akbar Haghdoost4 

 , Hadi Jabali2
, Hadi Jabali2 

 , Alireza Hajikhani5
, Alireza Hajikhani5 

 , Maryam Hoseini Abardeh6
, Maryam Hoseini Abardeh6 




 , Zeynab Foroughi2
, Zeynab Foroughi2 

 , Emad Karimian Rad *3
, Emad Karimian Rad *3 

 , Ali-Akbar Haghdoost4
, Ali-Akbar Haghdoost4 

 , Hadi Jabali2
, Hadi Jabali2 

 , Alireza Hajikhani5
, Alireza Hajikhani5 

 , Maryam Hoseini Abardeh6
, Maryam Hoseini Abardeh6 


1- Virtual School of Medical Education and Management, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2- Department of Health Service Management, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
3- Department of Health Service Management, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran ,emadkrad@gmail.com
4- Social Determinants of Health Research Center, Institute for Futures Studies in Health, Kerman, Iran
5- Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
6- National Agency for Strategic Research in Medical Education, Tehran. Iran
2- Department of Health Service Management, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran
3- Department of Health Service Management, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, Iran ,
4- Social Determinants of Health Research Center, Institute for Futures Studies in Health, Kerman, Iran
5- Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
6- National Agency for Strategic Research in Medical Education, Tehran. Iran
Keywords: Surveillance, COVID-19, Coronavirus, SARS-CoV-2, 2019-nCoV, Severe acute respiratory syndrome
Full-Text [PDF 1136 kb]
(379 Downloads)
| Abstract (HTML) (1846 Views)

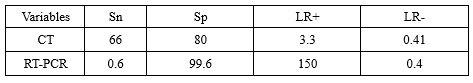
Accordingly, the simple clinical decision rule in Figure 1 will be used to determine the probability of COVID-19 infection.
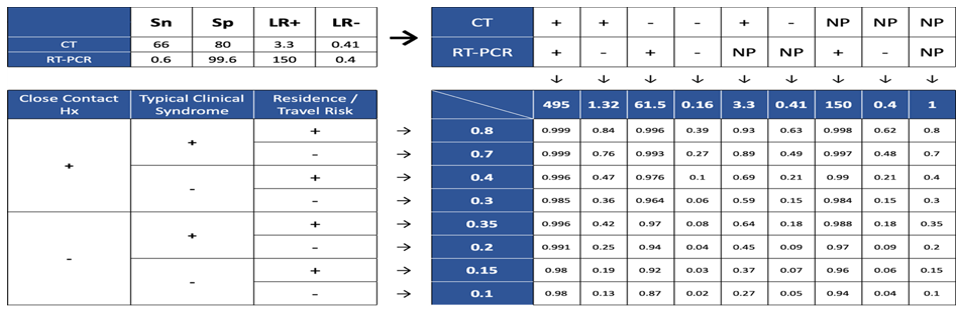
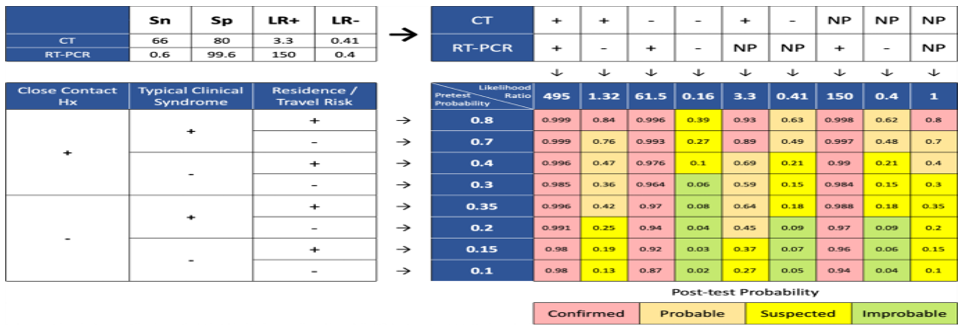
Third stage (Posttest probability): This stage aimed to modify and update the COVID-19 case definition by using the results of previous stages. In this stage, we tried to update the initial probability estimation. The likelihood ratio calculated with Diagnostic test results and the Pretest probability based on the existing knowledge was the input for calculating the posttest probability of covid-19. Consequently, the probability of the covid-19 disease infection is classified into the following four categories (Figure 2):
• Confirmed cases (Over 80% probability)
• Probable cases (Probability between 40% and 79%)
• Suspected cases (Probable between 10% and 39%)
• Improbable cases (Less than 10% probability)
Discussion
Heretofore, there was no definitive treatment for COVID-19. Therefore, a robust surveillance system is necessary for monitoring infected people (14). Furthermore, to achieve early detection and isolation of COVID-19 cases, careful assessments within the surveillance system are essential at primary healthcare centers (15). An appropriate case definition can contribute to the efficient identification of infected patients (11). Health authorities in different countries should revise their case definitions based on available epidemiological information (16).
Different countries' and organizations' COVID-19 case definitions were applied clinical criteria, including the most common symptoms of the disease and epidemiological criteria, including the history of travel or residence in a location with a report of community transmission of the disease and a history of close contact with a confirmed case. Based on our results, current case definitions only categorized and tested symptomatic patients as susceptible cases. However, studies showed that most people are asymptomatic carriers of the disease. It remains a huge challenge in disease prevention (17). Especially this challenge is prevalent among people under 15 and pregnant women. The results of a study on February 17, 2020, suggested among 1732 travelers, 189 were asymptomatic patients whose tests for new coronavirus were positive. Based on the result of this study, we should consider people with a history of close contact and travel to the affected regions for performing tests. (18).
We use the Bayesian approach for creating our case definition. The first use of the Bayes approach for disease diagnosis was by Homer Warner in 1961 (19). This version of Bayes’ formula is the mathematical equivalent of clinical diagnostic reasoning. We combine what we believe before doing the test with what we learn from the test to derive what we believe after doing the test. It makes use of the concepts of odds and likelihood ratio, which we define at this point. It also forms the basis for a simple pocket nomogram for rapidly working out post-test probabilities.
Based on our framework the possibility of a person from the affected region with typical clinical syndromes, normal chest CT, without a history of close contact, and in the absence of PCR test result, for COVID-19 is 18% and categorized as a suspected case (Figure 3).
Also, the possibility of a person from affected regions, without a history of close contact and disease symptoms, with normal chest CT and positive PCR for COVID-19 is 92% and categorized as a confirmed case (Figure 4).
The possibility of a person from a safe region, with history of close contact, in absence of disease symptoms, without chest CT, and with positive PCR is 98% and categorized as a confirmed case (Figure 5).
Conclusion
Currently, multiple diagnostic tests with multiple categories and the independency of the diagnostic tests are the challenges of covid-19 case definition, which by using the Bayesian approach this challenges wares. The decision to isolate and screen cases can be made based on the probability score obtained through this technique.
Acknowledgement
This study was funded and supported by the National Agency for Strategic Research in Medical Education (NASR). The Authors would like to thank all the participants in this study.
Funding sources
This study was funded and supported by the National Agency for Strategic Research in Medical Education (NASR).
Ethical statement
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Conflicts of interest
None.
Author contributions
ShY: Conceptualization, Methodology, Writing - Original Draft, ZF: Writing - Review and Editing EK: Writing - Review and Editing, Conceptualization, Methodology. AH and HJ and AH and MHA: Writing - Review and Editing All authors read and approved the final manuscript.
Full-Text: (526 Views)
Introduction
On December 31, 2019, China announced the emergence of a new type of coronavirus, which later on January 12, 2020, was named COVID-19 by the World Health Organization (WHO). COVID-19 rapidly spread across China and other countries. On March 11, 2020, WHO declared COVID-19 as a pandemic (1). As of 28 April 2020, 2,954,222 confirmed cases, with 202,597 deaths of COVID-19 have been reported to World Health Organization. COVID-19 infection presents vary from being asymptomatic to mild or severe pneumonia. Monitoring the number of coronavirus infections is necessary in providing an appropriate epidemiological response (2). Simple counting the number of COVID-19 cases can be misleading due to difficulties with patient access and performing laboratory tests. Therefore, evidence suggests the necessity of a robust surveillance systems to active people monitoring for disease infection. The International Health Regulation (IHR) defines surveillance as “the systematic ongoing collection, collation, and analysis of data for public health purposes and the timely dissemination of public health information for assessment and public health response as necessary” (2).
One of the first steps in response to the novel Coronavirus pandemic in several countries was strengthening of surveillance systems for identifying different cases. In this regard, countries enhanced their laboratory systems and defined cases that needed the test (3). Case definitions have been recognized as one of the most important components of public health surveillance systems (4). A case definition determines necessary and sufficient findings for classifying an individual as having a disease. More commonly, however, determining whether an individual has the disease is left to the expert judgment of a clinician (5). Case definitions assure the comparability and consistency of surveillance data. Applying case definitions considered essential by the WHO to make surveillance data comparable between countries. Transparent case definitions are necessary for effectively assessing an outbreak event. Applying a globally accepted case definition allows standardization of cases and provide comparisons inside and probably between outbreaks over time and in different geographical areas. Accepted case Definition includes defining and setting criteria for common demographic characteristics of Patients, location (Specific geographic area), time (Outbreak-related period), and clinical features (Clinical symptoms such as fever and cough) (6).
Case definitions can apply Boolean logical statements. These Definitions consist necessary and sufficient clinical findings with “AND” and “OR” operations. Also, case Definitions can use probabilistic statements that are based on conditional probabilities. In this approach, diagnosis will be categorized as confirmed, probable, or suspected. For example, If P (Disease | Data) > 0.99 the cases will be categorized as a confirmed case. (5). Therefore, this study suggests that it is more likely that a probabilistic approach can be more useful in COVID-19 case definitions.
Methods
This study was conducted in two phases. The first phase aimed at detecting different components, criteria, and categorizations of case definitions. Therefore, a rapid review of existing case definitions, was conducted. Also, a rapid review was conducted on the COVID-19 epidemiological studies. Also, to represent case definitions a dynamic case definition algorithm was applied using the Bayesian theorem models of diagnosis.
First phase: Rapid literature review
Search and databases
We conducted the review in two steps, in the first step a rapid review of existing case definitions conducted in PubMed and Google scholar from December 31, 2019, to April 3, 2020. Also, the Governmental websites of centers for disease control in different countries were searched to detect relevant document about countries case definitions. In this step, search strategy consisted of keyword that had similar meaning of “case definition” and “surveillance” along with “Corona-virus”, “Coronavirus”, “COVID-19”, “novel coronavirus”, “novel coronavirus disease”, “2019-nCoV”, “coronavirus disease”, “Severe acute respiratory syndrome” ,“coronavirus 2” and SARS-CoV-2.
In the second step, the rapid review was performed on epidemiological review studies of the new coronavirus disease published from December 31, 2019, to March 26, 2020. The aim was to achieve a comprehensive definition of various dimensions of Gregg's model, including the characteristics of person, place, time, and clinical features of the disease. In this step the keywords include, “Epidemiological characteristic”, “epidemiology”, “clinical features”, “incubation period”, “clinical description”, “clinical findings”, “clinical course”, symptoms, and surveillance, which used along with various new coronavirus keywords. The search strategy was conducted in Embase, PubMed and Google scholar databases.
Identified studies were screened by relevance of title and abstract and then full texts screened by inclusion and exclusion criteria.
Inclusion and exclusion criteria
Regarding case definitions, all types of evidence that provided case definitions of COVID-19, including reports of different countries and organizations were reviewed.
About the review of epidemiological studies, all English-language review articles that addressed the epidemiology of the new coronavirus disease, including clinical features, transmission methods, and demographic characteristics of COVID-19 cases were included. The studies that addressed the epidemiology of other coronaviruses or did not provide the required information excluded. Two researchers searched databases independently and two sets of studies compared and disagreements regarding the included studies resolved with discussion and third researcher’s opinion.
Data extraction
Based on Gregg's framework, all the information related to symptoms and diagnostic tests, including clinical features, disease-related periods (Incubation period, etc.), places of the disease transmission, and demographic and physical characteristics of patients, were extracted.
Second phase: Using the Bayesian approach
The Bayesian approach was used to create a dynamic case definition, with this purpose we combine disease likelihood (Data), including symptoms, signs, and findings of a patient with a pretest probability distribution of disease (Disease). Consequently, the probability of disease is P (Disease | Data). Variables in the Bayesian approach are diagnosis and findings used for diagnosing, it includes all findings which have high specificity to rule in and high sensitivity to rule out in diagnosis (7,8).
This approach was carried out in three steps: in the first step, the aim was to generate the pretest probability using the method of objective probability estimates. Second step, the aim was to gather more information about the COVID-19 disease and its diagnosis. Therefore, we focused on the studies related to diagnostic tests on COVID-19 cases. The aim of third step was to modify and update the covid-19 case definition by using the results of previous stages. In this stage, we tried to update the initial probability estimation. The input for calculating the posttest probability of COVID-19 was the likelihood ratio calculated with the results of diagnostic tests and the Pretest probability based on the existing knowledge.
Results
The majority of case definitions categorized as suspected, probable, and confirmed cases (Table 1).

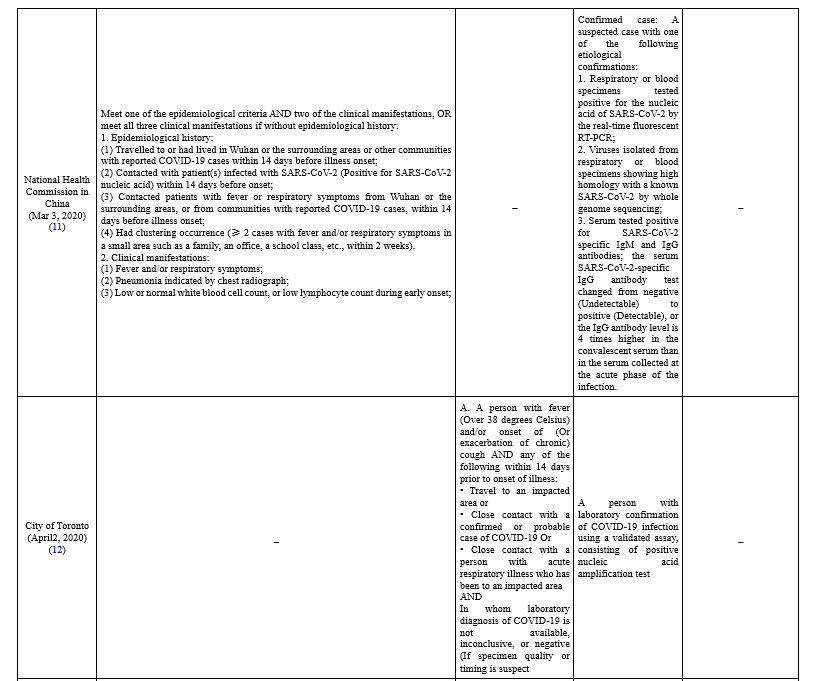
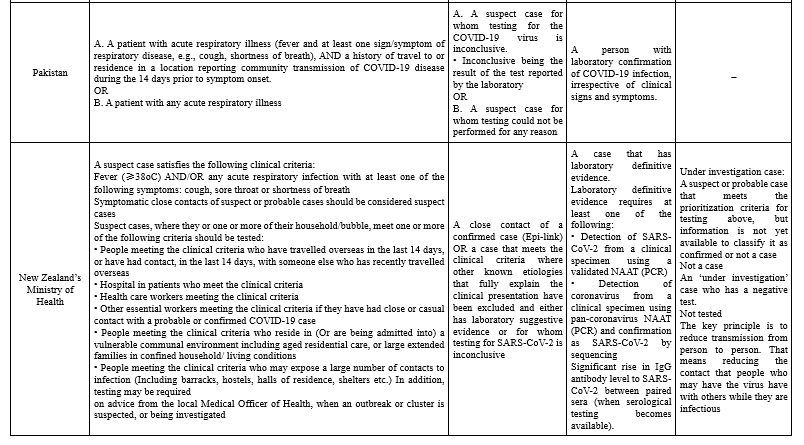
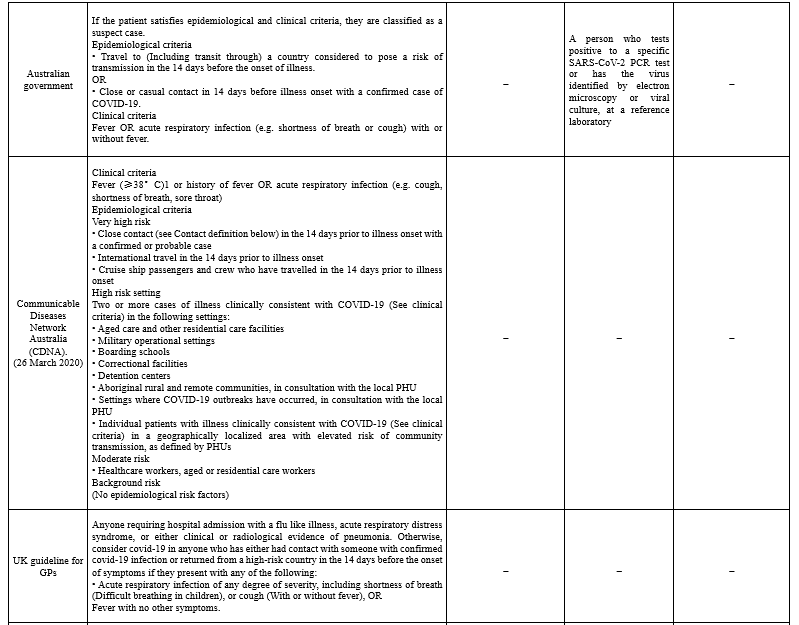

To define suspected cases, different organizations combined clinical criteria, which include the most common symptoms of COVID-19 patients (i.e. fever, cough, shortness of breath), with epidemiological criteria, including a history of travel to or residence in a location with reported community transmission of disease and a history of close contact with a confirmed case. In Taiwan, case definition classification is divided into reporting, probable, and confirmed cases. Patients with one of the clinical criteria and epidemiological criteria or one laboratory criteria are classified as reporting cases (10). In New Zealand, suspected cases are prioritized based on having some criteria which they or one or more of their household/bubble have for the test (13). The communicable Diseases Network in Australia (CDNA) divided suspected cases into Very high risk, High-risk setting, Moderate risk, and Background risk based on their epidemiologic criteria.
The definition's parameters are not conclusive in the Coronavirus literature because of the lack of available data. Data on prevalence, for example, is obtained from RT-PCR positive results of testing for the coronavirus, and tests have been rationed and almost entirely have been administered to a selected population mainly patients presenting with severe symptoms or vulnerable individuals. Thus, the fraction of positive test results does not reflect the total infection rate of the population. Although these tests were made available during the outbreak, their sensitivity and specificity were unknown because there are no “gold standard” laboratory or clinical definitions for the diagnosis of COVID-19. The disease definitions developed by the World Health Organization (WHO), the US Centers for Disease Control and Prevention, European Centre for Disease Prevention and Control (ECDC) care are broadly inclusive and nonspecific.
Also, data extracted from 10 epidemiological review studies in four dimensions of person, time, place, and clinical features showed that an important parameter is the asymptomatic individual rate (The fraction of the infected who are not tested under current guidelines). Therefore, estimation lacks in the literature because tests for the coronavirus have been targeted at the sick and vulnerable. However, a number of studies suggested an estimation by a general screening of the population. Furthermore, various studies showed the susceptibility and vulnerability of people to disease based on a variety of underlying conditions such as old age, hypertension, diabetes, cardiovascular diseases, immunosuppressive diseases, and male gender.
Following is our finding based on the literature review and using the Bayesian method.
First stage (Pretest probability)
By reviewing existing studies the pretest probability was generated based on three criteria:
(i) The presence or absence of a history of close contact with the COVID-19 case. Based on the result of the literature review, in this study close contact include:
• Living with a confirmed COVID-19 case
• Direct contact without protective equipment or contact with bodily fluids of a confirmed covid-19 case individual
• Direct or face to face contact with a confirmed COVID-19 case individual regardless of time
• Being less than two meters away from a confirmed COVID-19 case individual for more than 15 minutes
• Referral and visit to a medical center where people with COVID-19 have been admitted.
• Health care staff dealing with COVID-19
• Being otherwise advised by a public health agency that contact with a confirmed case has occurred
(ii) The presence or absence of typical clinical syndrome which respectively includes: Fever, dry cough, myalgia, fatigue, and normal or decreased white blood cells and decreased lymphocytes at the onset of the disease
(iii) History of Living in or traveling to an area with a high incidence or prevalence of COVID-19 case (An area that accounts for more than 30% of community infections).
Second stage (Likelihood ratio)
Evidence suggested that two medical tests seem to be beneficial in covid-19 diagnosis which were CT-Scan of lung and RT-PCR. Based on this result these two tests could be a key parameter to achieve a better understanding of COVID-19 case definition. To measure the validity of these two tests, several studies were identified and reviewed leading to determining their specificity (Sp) and sensitivity (Sn), consequently the “Likelihood ratio” was calculated for CT-Scan and RT-PCR. The mentioned tests likelihood ratio was figured in the following terms:
CT scan of the lung
• Positive result (+)
• Negative result (-)
• Not-performed (NP) or inconclusive results
RT-PCR test
• Positive result (+)
• Negative result (-)
Not-performed (NP)
Post-test probability was calculated as follows (Table 2):
On December 31, 2019, China announced the emergence of a new type of coronavirus, which later on January 12, 2020, was named COVID-19 by the World Health Organization (WHO). COVID-19 rapidly spread across China and other countries. On March 11, 2020, WHO declared COVID-19 as a pandemic (1). As of 28 April 2020, 2,954,222 confirmed cases, with 202,597 deaths of COVID-19 have been reported to World Health Organization. COVID-19 infection presents vary from being asymptomatic to mild or severe pneumonia. Monitoring the number of coronavirus infections is necessary in providing an appropriate epidemiological response (2). Simple counting the number of COVID-19 cases can be misleading due to difficulties with patient access and performing laboratory tests. Therefore, evidence suggests the necessity of a robust surveillance systems to active people monitoring for disease infection. The International Health Regulation (IHR) defines surveillance as “the systematic ongoing collection, collation, and analysis of data for public health purposes and the timely dissemination of public health information for assessment and public health response as necessary” (2).
One of the first steps in response to the novel Coronavirus pandemic in several countries was strengthening of surveillance systems for identifying different cases. In this regard, countries enhanced their laboratory systems and defined cases that needed the test (3). Case definitions have been recognized as one of the most important components of public health surveillance systems (4). A case definition determines necessary and sufficient findings for classifying an individual as having a disease. More commonly, however, determining whether an individual has the disease is left to the expert judgment of a clinician (5). Case definitions assure the comparability and consistency of surveillance data. Applying case definitions considered essential by the WHO to make surveillance data comparable between countries. Transparent case definitions are necessary for effectively assessing an outbreak event. Applying a globally accepted case definition allows standardization of cases and provide comparisons inside and probably between outbreaks over time and in different geographical areas. Accepted case Definition includes defining and setting criteria for common demographic characteristics of Patients, location (Specific geographic area), time (Outbreak-related period), and clinical features (Clinical symptoms such as fever and cough) (6).
Case definitions can apply Boolean logical statements. These Definitions consist necessary and sufficient clinical findings with “AND” and “OR” operations. Also, case Definitions can use probabilistic statements that are based on conditional probabilities. In this approach, diagnosis will be categorized as confirmed, probable, or suspected. For example, If P (Disease | Data) > 0.99 the cases will be categorized as a confirmed case. (5). Therefore, this study suggests that it is more likely that a probabilistic approach can be more useful in COVID-19 case definitions.
Methods
This study was conducted in two phases. The first phase aimed at detecting different components, criteria, and categorizations of case definitions. Therefore, a rapid review of existing case definitions, was conducted. Also, a rapid review was conducted on the COVID-19 epidemiological studies. Also, to represent case definitions a dynamic case definition algorithm was applied using the Bayesian theorem models of diagnosis.
First phase: Rapid literature review
Search and databases
We conducted the review in two steps, in the first step a rapid review of existing case definitions conducted in PubMed and Google scholar from December 31, 2019, to April 3, 2020. Also, the Governmental websites of centers for disease control in different countries were searched to detect relevant document about countries case definitions. In this step, search strategy consisted of keyword that had similar meaning of “case definition” and “surveillance” along with “Corona-virus”, “Coronavirus”, “COVID-19”, “novel coronavirus”, “novel coronavirus disease”, “2019-nCoV”, “coronavirus disease”, “Severe acute respiratory syndrome” ,“coronavirus 2” and SARS-CoV-2.
In the second step, the rapid review was performed on epidemiological review studies of the new coronavirus disease published from December 31, 2019, to March 26, 2020. The aim was to achieve a comprehensive definition of various dimensions of Gregg's model, including the characteristics of person, place, time, and clinical features of the disease. In this step the keywords include, “Epidemiological characteristic”, “epidemiology”, “clinical features”, “incubation period”, “clinical description”, “clinical findings”, “clinical course”, symptoms, and surveillance, which used along with various new coronavirus keywords. The search strategy was conducted in Embase, PubMed and Google scholar databases.
Identified studies were screened by relevance of title and abstract and then full texts screened by inclusion and exclusion criteria.
Inclusion and exclusion criteria
Regarding case definitions, all types of evidence that provided case definitions of COVID-19, including reports of different countries and organizations were reviewed.
About the review of epidemiological studies, all English-language review articles that addressed the epidemiology of the new coronavirus disease, including clinical features, transmission methods, and demographic characteristics of COVID-19 cases were included. The studies that addressed the epidemiology of other coronaviruses or did not provide the required information excluded. Two researchers searched databases independently and two sets of studies compared and disagreements regarding the included studies resolved with discussion and third researcher’s opinion.
Data extraction
Based on Gregg's framework, all the information related to symptoms and diagnostic tests, including clinical features, disease-related periods (Incubation period, etc.), places of the disease transmission, and demographic and physical characteristics of patients, were extracted.
Second phase: Using the Bayesian approach
The Bayesian approach was used to create a dynamic case definition, with this purpose we combine disease likelihood (Data), including symptoms, signs, and findings of a patient with a pretest probability distribution of disease (Disease). Consequently, the probability of disease is P (Disease | Data). Variables in the Bayesian approach are diagnosis and findings used for diagnosing, it includes all findings which have high specificity to rule in and high sensitivity to rule out in diagnosis (7,8).
This approach was carried out in three steps: in the first step, the aim was to generate the pretest probability using the method of objective probability estimates. Second step, the aim was to gather more information about the COVID-19 disease and its diagnosis. Therefore, we focused on the studies related to diagnostic tests on COVID-19 cases. The aim of third step was to modify and update the covid-19 case definition by using the results of previous stages. In this stage, we tried to update the initial probability estimation. The input for calculating the posttest probability of COVID-19 was the likelihood ratio calculated with the results of diagnostic tests and the Pretest probability based on the existing knowledge.
Results
The majority of case definitions categorized as suspected, probable, and confirmed cases (Table 1).





To define suspected cases, different organizations combined clinical criteria, which include the most common symptoms of COVID-19 patients (i.e. fever, cough, shortness of breath), with epidemiological criteria, including a history of travel to or residence in a location with reported community transmission of disease and a history of close contact with a confirmed case. In Taiwan, case definition classification is divided into reporting, probable, and confirmed cases. Patients with one of the clinical criteria and epidemiological criteria or one laboratory criteria are classified as reporting cases (10). In New Zealand, suspected cases are prioritized based on having some criteria which they or one or more of their household/bubble have for the test (13). The communicable Diseases Network in Australia (CDNA) divided suspected cases into Very high risk, High-risk setting, Moderate risk, and Background risk based on their epidemiologic criteria.
The definition's parameters are not conclusive in the Coronavirus literature because of the lack of available data. Data on prevalence, for example, is obtained from RT-PCR positive results of testing for the coronavirus, and tests have been rationed and almost entirely have been administered to a selected population mainly patients presenting with severe symptoms or vulnerable individuals. Thus, the fraction of positive test results does not reflect the total infection rate of the population. Although these tests were made available during the outbreak, their sensitivity and specificity were unknown because there are no “gold standard” laboratory or clinical definitions for the diagnosis of COVID-19. The disease definitions developed by the World Health Organization (WHO), the US Centers for Disease Control and Prevention, European Centre for Disease Prevention and Control (ECDC) care are broadly inclusive and nonspecific.
Also, data extracted from 10 epidemiological review studies in four dimensions of person, time, place, and clinical features showed that an important parameter is the asymptomatic individual rate (The fraction of the infected who are not tested under current guidelines). Therefore, estimation lacks in the literature because tests for the coronavirus have been targeted at the sick and vulnerable. However, a number of studies suggested an estimation by a general screening of the population. Furthermore, various studies showed the susceptibility and vulnerability of people to disease based on a variety of underlying conditions such as old age, hypertension, diabetes, cardiovascular diseases, immunosuppressive diseases, and male gender.
Following is our finding based on the literature review and using the Bayesian method.
First stage (Pretest probability)
By reviewing existing studies the pretest probability was generated based on three criteria:
(i) The presence or absence of a history of close contact with the COVID-19 case. Based on the result of the literature review, in this study close contact include:
• Living with a confirmed COVID-19 case
• Direct contact without protective equipment or contact with bodily fluids of a confirmed covid-19 case individual
• Direct or face to face contact with a confirmed COVID-19 case individual regardless of time
• Being less than two meters away from a confirmed COVID-19 case individual for more than 15 minutes
• Referral and visit to a medical center where people with COVID-19 have been admitted.
• Health care staff dealing with COVID-19
• Being otherwise advised by a public health agency that contact with a confirmed case has occurred
(ii) The presence or absence of typical clinical syndrome which respectively includes: Fever, dry cough, myalgia, fatigue, and normal or decreased white blood cells and decreased lymphocytes at the onset of the disease
(iii) History of Living in or traveling to an area with a high incidence or prevalence of COVID-19 case (An area that accounts for more than 30% of community infections).
Second stage (Likelihood ratio)
Evidence suggested that two medical tests seem to be beneficial in covid-19 diagnosis which were CT-Scan of lung and RT-PCR. Based on this result these two tests could be a key parameter to achieve a better understanding of COVID-19 case definition. To measure the validity of these two tests, several studies were identified and reviewed leading to determining their specificity (Sp) and sensitivity (Sn), consequently the “Likelihood ratio” was calculated for CT-Scan and RT-PCR. The mentioned tests likelihood ratio was figured in the following terms:
CT scan of the lung
• Positive result (+)
• Negative result (-)
• Not-performed (NP) or inconclusive results
RT-PCR test
• Positive result (+)
• Negative result (-)
Not-performed (NP)
Post-test probability was calculated as follows (Table 2):

Accordingly, the simple clinical decision rule in Figure 1 will be used to determine the probability of COVID-19 infection.
Third stage (Posttest probability): This stage aimed to modify and update the COVID-19 case definition by using the results of previous stages. In this stage, we tried to update the initial probability estimation. The likelihood ratio calculated with Diagnostic test results and the Pretest probability based on the existing knowledge was the input for calculating the posttest probability of covid-19. Consequently, the probability of the covid-19 disease infection is classified into the following four categories (Figure 2):
• Confirmed cases (Over 80% probability)
• Probable cases (Probability between 40% and 79%)
• Suspected cases (Probable between 10% and 39%)
• Improbable cases (Less than 10% probability)
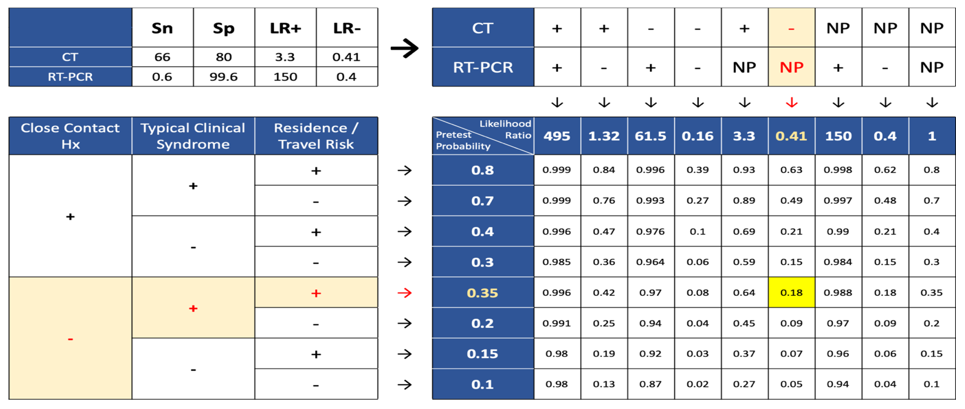 Figure 3. Post-test probability for CT (-), RT-PCR (NP), close contact (-), symptom (+), location (+) 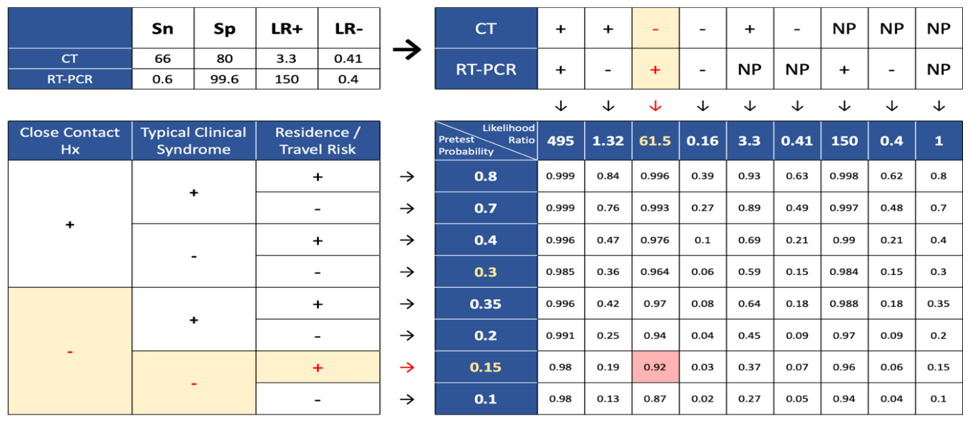 Figure 4. Post-test probability for CT (-), RT-PCR (+), close contact (-), symptom (-), location (+) 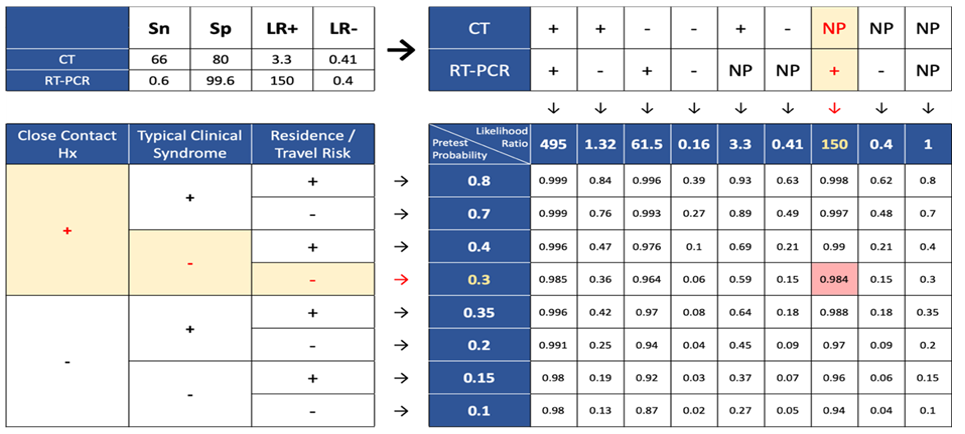 Figure 5. Post-test probability for CT (NP), RT-PCR (+), close contact (+), symptom (-), location (-) |
Discussion
Heretofore, there was no definitive treatment for COVID-19. Therefore, a robust surveillance system is necessary for monitoring infected people (14). Furthermore, to achieve early detection and isolation of COVID-19 cases, careful assessments within the surveillance system are essential at primary healthcare centers (15). An appropriate case definition can contribute to the efficient identification of infected patients (11). Health authorities in different countries should revise their case definitions based on available epidemiological information (16).
Different countries' and organizations' COVID-19 case definitions were applied clinical criteria, including the most common symptoms of the disease and epidemiological criteria, including the history of travel or residence in a location with a report of community transmission of the disease and a history of close contact with a confirmed case. Based on our results, current case definitions only categorized and tested symptomatic patients as susceptible cases. However, studies showed that most people are asymptomatic carriers of the disease. It remains a huge challenge in disease prevention (17). Especially this challenge is prevalent among people under 15 and pregnant women. The results of a study on February 17, 2020, suggested among 1732 travelers, 189 were asymptomatic patients whose tests for new coronavirus were positive. Based on the result of this study, we should consider people with a history of close contact and travel to the affected regions for performing tests. (18).
We use the Bayesian approach for creating our case definition. The first use of the Bayes approach for disease diagnosis was by Homer Warner in 1961 (19). This version of Bayes’ formula is the mathematical equivalent of clinical diagnostic reasoning. We combine what we believe before doing the test with what we learn from the test to derive what we believe after doing the test. It makes use of the concepts of odds and likelihood ratio, which we define at this point. It also forms the basis for a simple pocket nomogram for rapidly working out post-test probabilities.
Based on our framework the possibility of a person from the affected region with typical clinical syndromes, normal chest CT, without a history of close contact, and in the absence of PCR test result, for COVID-19 is 18% and categorized as a suspected case (Figure 3).
Also, the possibility of a person from affected regions, without a history of close contact and disease symptoms, with normal chest CT and positive PCR for COVID-19 is 92% and categorized as a confirmed case (Figure 4).
The possibility of a person from a safe region, with history of close contact, in absence of disease symptoms, without chest CT, and with positive PCR is 98% and categorized as a confirmed case (Figure 5).
Conclusion
Currently, multiple diagnostic tests with multiple categories and the independency of the diagnostic tests are the challenges of covid-19 case definition, which by using the Bayesian approach this challenges wares. The decision to isolate and screen cases can be made based on the probability score obtained through this technique.
Acknowledgement
This study was funded and supported by the National Agency for Strategic Research in Medical Education (NASR). The Authors would like to thank all the participants in this study.
Funding sources
This study was funded and supported by the National Agency for Strategic Research in Medical Education (NASR).
Ethical statement
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Conflicts of interest
None.
Author contributions
ShY: Conceptualization, Methodology, Writing - Original Draft, ZF: Writing - Review and Editing EK: Writing - Review and Editing, Conceptualization, Methodology. AH and HJ and AH and MHA: Writing - Review and Editing All authors read and approved the final manuscript.
Type of Study: Original Article |
Subject:
Others
Received: 2022/03/12 | Accepted: 2022/05/29 | Published: 2024/07/9
Received: 2022/03/12 | Accepted: 2022/05/29 | Published: 2024/07/9
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |